ConnectHEOR hosted a webinar on 18th January 2022 by Professor Egon Jonsson on the topic “Health technology assessment: A historical ride and present story”. This blog captures the key points of the discussion in the webinar.
Professor Jonsson is a ConnectHEOR expert and professor at University of Alberta and Calgary in Canada. He is a co-founder of HTAi, its Society, and its scientific journal-The International Journal of Technology Assessment in Health Care. In addition, he was the CEO of Swedish Agency for Health Technology Assessment (SBU.se), the Leader of the WHO Health Evidence Network (WHO. Int.Hen), and the CEO of the Institute of Health Economics in Edmonton, Canada (IHE.ca).
Health Technology Assessment (HTA) evaluates the medical, social, ethical, legal, organizational, and economic implications of technology, including drugs, devices, medical and surgical procedures. The HTA is a multidisciplinary form of scientific research to synthesize evidence for the support of health policy and practice.
Brief History on Formation and Development of HTA:
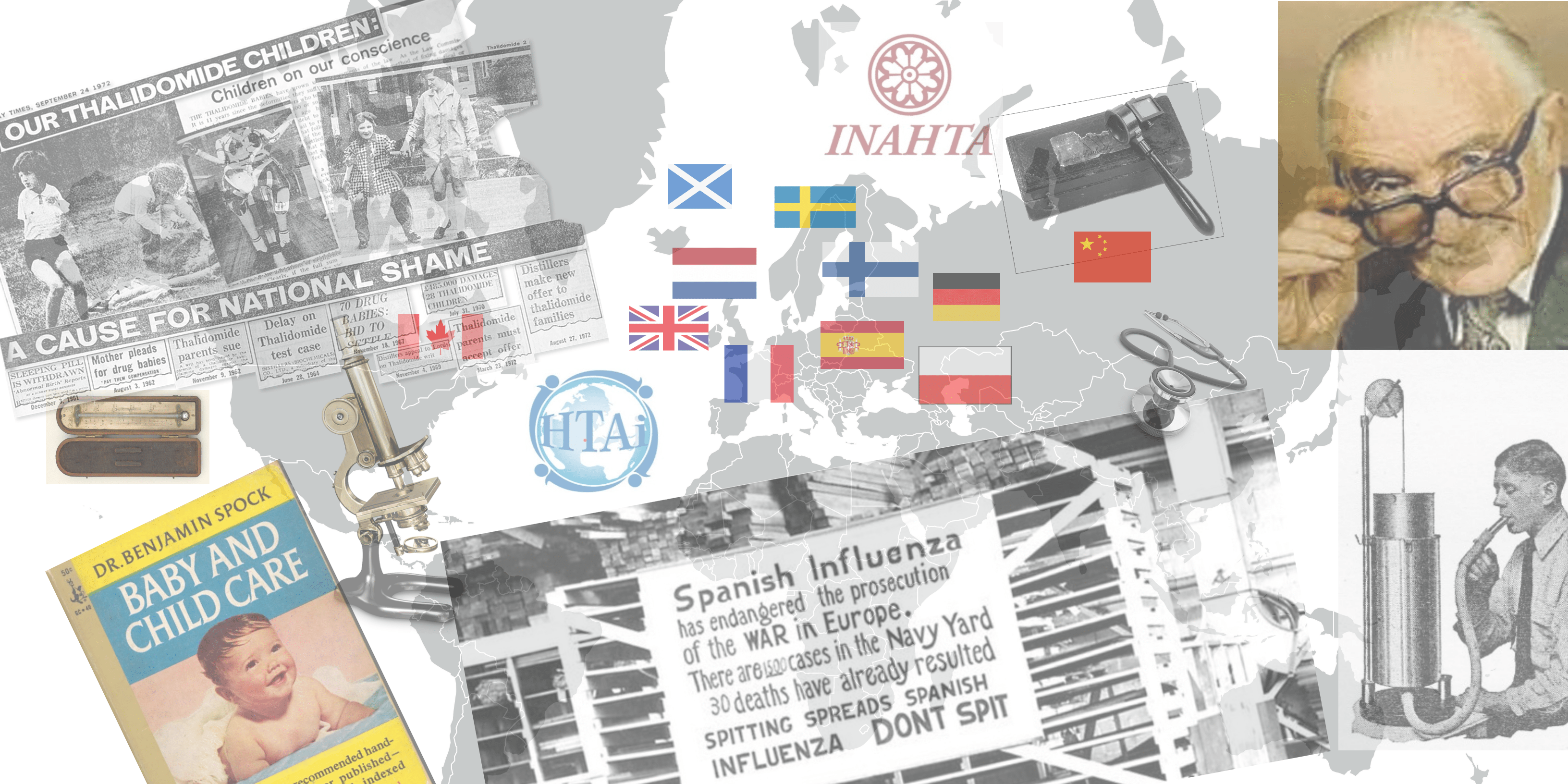
The first known documented efforts to collect data and use some kind of evidence to improve healthcare is from 350 years ago when the Swedish Collegium Medicorum was established to: distinguish quackery from medicine, control the trade of poisonous drugs, and banish all swindlers who “grease people with their fake, fraudulent and harmful medicaments”.
Some notable discoveries and technologies in the medicine in the 1800s were: microscope (1695), vaccine (1796), stethoscope (1816), spirometer (1840), ophthalmoscope (1850), the cell (1858), thermometer (1866) and X-Ray (1895).
The US Congressional Office of Technology Assessment (OTA) was established 1972 to support the Congress with assessments suitable for policy making such as space, transportation, agricultural and environmental technologies. Later, health Technologies were added to the program in 1974.
There were some historic events which increased the interest in establishing HTA: horrific treatments (Thalidomide Scandal and Lobotomy), opinion-based medicine (which was eventually replaced by evidence based medicine), the publication of Archie Cochranes landmark “Effectiveness and Efficiency in the Health Services” and the introduction of a stunning technology developed by EMI in the UK (CT scanner). Interestingly, the CT scanner and Cochrane marked the very beginning of HTA in 1974.
For evaluation of the CT scanner, the policy makers demanded a synthesis of what are the medical, ethical, organizational, social, and economic implications of this technology along with a thorough comparison of the alternatives. The research community felt that many questions can be answered by a more structured approach combining safety (what is the risk of the technology known), efficacy (is the technology doing what it is supposed to do), effectiveness (how does it impact patient health health) and cost-effectiveness (what are the costs related to health outcomes).
The program at OTA inspired many researchers outside Europe. The first agency outside the US was established in Sweden in the 1980s, followed by 14 more agencies in Europe within a period of about 10 years (in countries: Spain, France, Holland, Belgium, Canada, Poland, United Kingdom, Scotland, Germany, Finland and Norway). Eventually agencies were established in Australia, South America, Turkey, Malaysia, Thailand, China, South Korea and South Africa. INAHTA was formed in 1993 at a meeting in Paris and has about 40 member agencies.
There were some other developments of importance that synergised HTA such as: an international society (ISTAHC) and a scholarly journal with CUP in 1985, databases of scientific studies, software and skills to retrieve and synthesize information, personal computers and internet, sustainable demand for HTAs and political and academic support.
The discussion also briefly touched upon the Health Evidence Network (HEN) by World Health Organization (WHO) which is not covered in this blog.
Present state and trends in HTA
Since its development, HTA is used to assist in health policy decision making, controlling the diffusion and use of technology, reimbursement decisions, development of guidelines and a basis for innovations in health services.
HTA is based on findings from scientific studies on the safety, efficacy, effectiveness and cost-effectiveness of both new and established technologies in health care. It has a strong political support in most countries and is known for providing syntheses of findings from research useful in health policy making and practice.
HTAs are produced and used by both Governments, Academia and Industry. There are currently 52 agencies in more than 30 countries who are members of INAHTA. There are an estimated 15 more agencies in HTA. Together with researchers working on HTAs in universities, industry, and in the consulting sector there are an estimated 5,000-10,000 researchers in this field.
Now-a-days for HTA, there is a trend towards specialized groups doing assessment of specific drugs and devices, broader policy issues being raised more frequently and comparative effectiveness most likely incorporating both HTA and Evidence Based Medicine.
When assessing or conducting a HTA, to have a holistic view, there can be ten most important issues to address:
What are the benefits to the patients of a new or already established technology in healthcare?
What are the alternatives to the investment?
What are the broader economic, social and equity implications of the investment?
Is the investment affordable and cost-effective?
Is management and clinical skill available for appropriate use and development of the technology?
Is the technology safe, effective and cost-effective?
Are training and maintenance secured?
What are the potential social, and equity implications of the diffusion of the technology?
Will the indication for use of the technology expand?
What are the impacts of the technology on access to health services, fees, waiting times, and insurance issues?
Questions & Answers
Q1:How do we expect the EU market to change after the adoption of EU regulation on joint HTAs?
The EU regulation on HTA was adopted in December 2021 and will apply from January 2025. The following text is based on the adopted proposal published by the Commission.
During the next 3 years, the Commission will be setting up the necessary governance structure and preparatory documents to ensure effective application. The Commission will invite Member States to nominate their members of a Coordination Group, which is expected to have their first meeting mid-2022 and begin to; establish the Stakeholder Network adopt the necessary implementing and delegated acts facilitate the development of methodology for joint HTA work, joint scientific consultations, the identification of emerging health technologies, work on joint clinical assessments.
For HTAs there will be a single EU-level submission file for Joint Clinical Assessments instead of multiple parallel submissions to the different national HTA systems. HTAs will assess the relative safety and effectiveness of new health technology as compared with existing technologies. They will also engage in Joint Scientific Consultations to advise technology developers on clinical study designs that generate appropriate evidence. Moreover, “horizon scanning” exercises will identify, at an early stage, promising health technologies.
Developers in the pharmaceutical and medical device sectors will have more clarity and predictability concerning the clinical evidence requirements for HTA. It is of importance to note, however, that the Member States will remain responsible for drawing conclusions on the overall value of new health technology for their healthcare system, and of pricing and reimbursement decisions. The Coordination Group will need to deal with quite a few issues, including many details and definitions. Until this is done it would be too early to draw conclusions about if and how the EU market possibly will change.
It is of importance to note, however, that the Member States will remain responsible for drawing conclusions on the overall value of new health technology for their healthcare system, and decide on pricing and reimbursement.
Q2: What’s your thought on ensuring wider awareness and uptake of HTA and related analyses in healthcare decisions?
First and foremost publish your results in a peer-reviewed Journal. The following recommendations are based on experience and some evidence from different types of studies:
Develop a strategy to reach clinicians with your findings, particularly those in primary healthcare since most others are specialists and therefore likely being updated on progress within their discipline. As far as possible make use of highly respected people as “Ambassadors” who were also members of your assessment teams.
Develop strategies to reach also the general public about the results of your HTAs. For example, by appointing one member of each of your assessment projects to draft a press release of your outcomes and invite journalists to a press conference focused on a specific finding of particular interest to the public.
Develop also a lay version of the issue and the outcomes of the assessments and distribute these as brochures, free of charge and available at pharmacies. The mass media and the public will become strong supporters of HTAs produced by you.
Q3: Is opinion-based medicine different from evidence-based medicine?
In short: Yes.
Evidence-Based Medicine (EBM) is about how to best treat individual patients while HTAs mainly is for policymakers to regulate the diffusion and use of technology. A common definition of EBM is as follows; “the conscientious, explicit and judicious use of current best evidence in making decisions about the care of individual patients”
Q4:Do you think artificial intelligence has any application in HTA and how that will change the dynamics?
I strongly believe that AI almost certainly will have several applications in any HTA. The most likely dynamics will be apparent in a much shorter time frame for completing an HTA and that it will deal with potential bias better than humans.
Q5: I don’t think I recall you mentioning the QALYs once – do you feel we measure QALYs well enough given its importance in decision making?
There was no intention of mine to not mention QALYs. I am truly sorry I did not bring it up. I could have done it in connection to the case of the CT scanner: a formidable example of how a new technology almost overnight made several types of examinations redundant. Some of the older examinations often caused suffering, anxiety, fear, and a high risk of complications, even death, especially in patients with suspected brain tumors. CT did it with much higher accuracy and almost perfect quality, at a fraction of the cost of the traditional examinations. It is self-evident that quality of life may be affected in different directions by new technology and therefore need to be measured and included in the syntheses and recommendations of HTAs.
When I read Milton Weinstein and Bill Stason’s classic on Hypertension: A policy perspective in the mid-1970s where they suggested that QALYs (which had been coined by other researchers long before) could be measured by anyone facing the question; “Taking into account your age, pain, immobility, etc what fraction of a year of life would you be willing to give up to be completely healthy the remaining fraction of a year instead of your present level of health status for the full year”. I along with one of my associates constructed a set of fictional health statuses due to hypertension, stroke, and MI and used the Weinstein/Stason proposal in interviews of three groups; physicians, students, and a random sample of people representing the general public. It turned out that physicians were not willing to give up much, while the other two groups ranked it as being more serious.
Incidentally, two of our respondents, twins and young men, responded by saying that they would both be willing to give up at least one month, namely November. When we presented our study results for a group of about 30 physicians we were almost totally disregarded and many said it straightforward that this measure was not anything for them to be concerned about. This was in the early 1980s. Now it is clearly different. Quality of life must be part of any HTA. This is partly due to the works of the nice and very wise economist Alan Williams in the UK, who made the concept acceptable and easy to understand not the least by policymakers, first in the UK Government, later in many jurisdictions around the word. Policymakers in many countries had for a long time looking for just the type of measure that he proposed. Once again, QALYs are indeed important to measure in HTAs.
Q6: What do you think about the transferability and generalizability of HTA across jurisdictions? Is it possible?
In principle, the findings from HTAs may be transferred between jurisdictions. However, in practice cultural, social, population characteristics, affordability, and changes in such factors may make it difficult. If we speak only about certain aspects of HTA, such as safety and efficacy it would seem to be feasible and even eligible to make the findings transferable.
Q7: Any advice for establishing a new HTA?
Yes. Do not expect immediate results of a proposal to establish a new agency for HTAs. It will take some time. I would suggest that you, first of all, seek the support of highly regarded individuals in healthcare; clinical researchers, economists, management, administration, organization, ethics by explaining the essence of HTAs, what they can contribute with; higher safety, efficacy, effectiveness and better cost-effectiveness. That quality of care can and will improve with HTA and that it is possible to reduce the speed of increasing cost of health services. HTA is actually one of the few currently known measures that have that capability. Eventually, broaden your circle of champions for HTA and seek to get political or health insurance support for establishing a new agency or a department at a university or an independent institute of HTA.
There is a special issue of the International Journal of Health Technology Assessment named History of HTA, which contains descriptions of how individual researchers in many countries managed to get, often high-level policy decision-makers, to be interested in the idea of an agency or other constellation for HTA, and it also provides insights into how international collaboration and professional societies grew up. This issue of the Journal is about 300 pages but well worth reading to get more advice than I can provide here.
Q8: As a young researcher in HTA, I wish to move into research on HTA in low to middle-income countries. Do you have any advice for orienting oneself in this topic?
This is something that is missing in the world. The poorest countries have the greatest need for good advice about making any investment in the field of healthcare, whether technology or for prevention of diseases, mental health issues, etc. I would think that you could seek good advice, support, and perhaps even some funding by approaching WHO headquarters, the GD himself, or any of WHOs country offices. Try to get them to be supportive and collaborative in approaching international organizations such as the Rockefeller Foundation, which has helped some low income countries with funding to get started. Bill and Melinda Gates foundation could also be interested in supporting you on this. Start anywhere possibly feasible. It may take you to where you want to be with HTA.
Q9: What is the scenario of reimbursement in digital health technology? Are digital health technologies reimbursed?
I am not very knowledgeable in the field of digital health solutions. However, it seems that some European countries are more active than others, for example, Sweden, Germany, and the UK. Germany is exploring which of its many reimbursement mechanisms fit for which type of DHS. Also public and private health insurance companies already have decided to reimburse digital health technologies based on safety, efficacy, and privacy conditions. To some extent, the producers face the same requirements as for the pharma and medical device industries with HTAs, although for DHS safety and robust high-quality data is at the forefront, while universal reimbursement seem to be somewhat far into the future.
Authors – Prof. Egon Jonsson, Tushar Srivastava & Radha Shukla
To avail complete webinar resources click here.
Discussion forum in comments:
Do you have any pending questions or discussion items? Add them in the comments!













2 Responses
This interefview is very stimulating, since there are only a few peoples, who can descrbe the actual history of HTA.
Anyway, I am trying to contact with Egon. However, the past e-mail address was not active.
Please let me konw his recent e-mail address.
Thank you for your consideration.
Please let me konw Egon’s e-mail address. Recently, I can not contact with him.